Sae End Date Start date First day with any adverse symptoms or a clear deterioration of a pre existing condition For example the onset of an event may occur before a hospitalisation Stop date
The clinical team creates the SAE visit in CTMS within 24 hours of notification of the SAE When Research Financial Operations is notified that an SAE is related to a drug or device that notification begins the SAE tracking Record the date of resolution in DD MMM YYYYformat If the SAE is ongoing at the end of the study check the Ongoing at end of study box
Sae End Date
Sae End Date
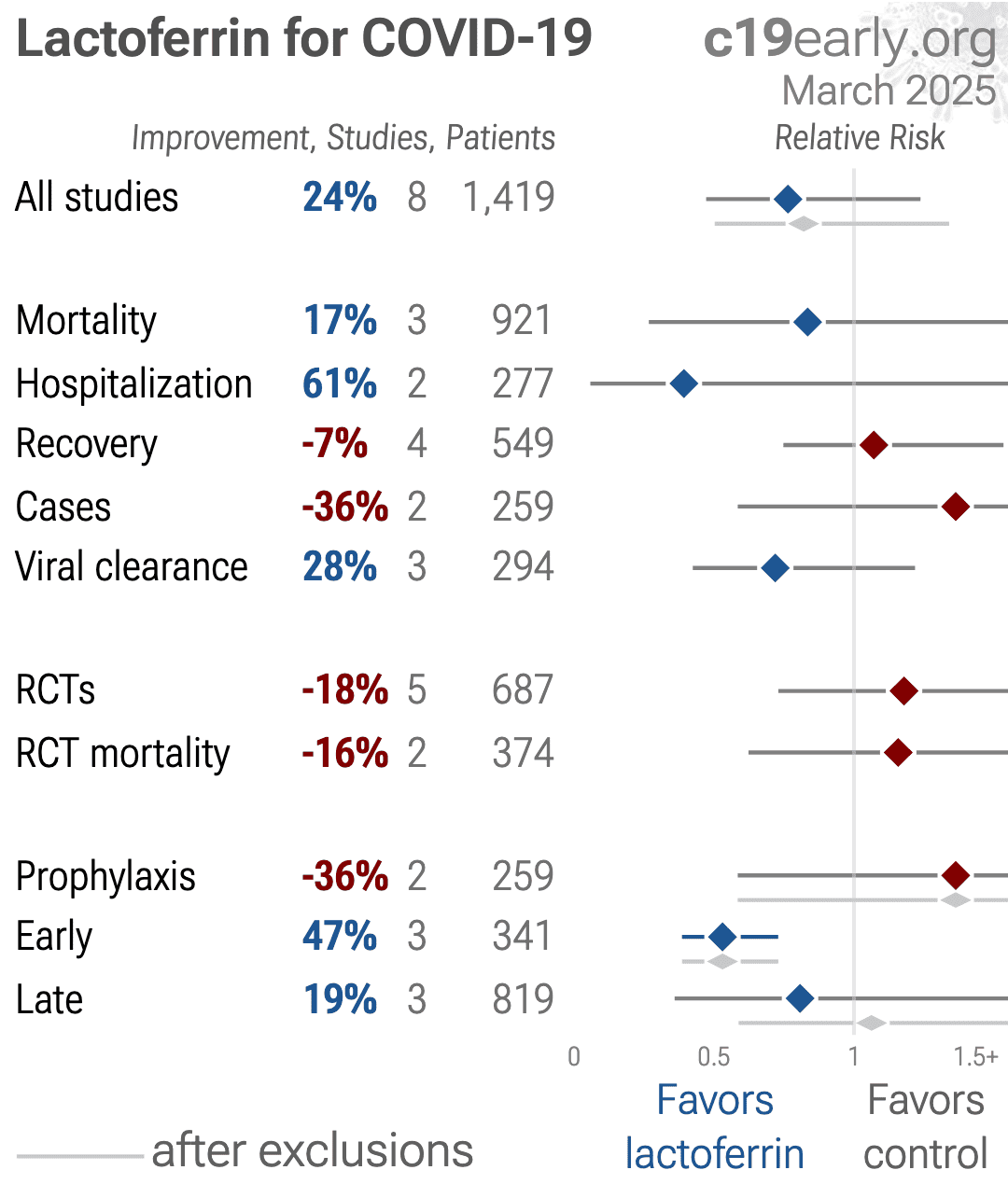
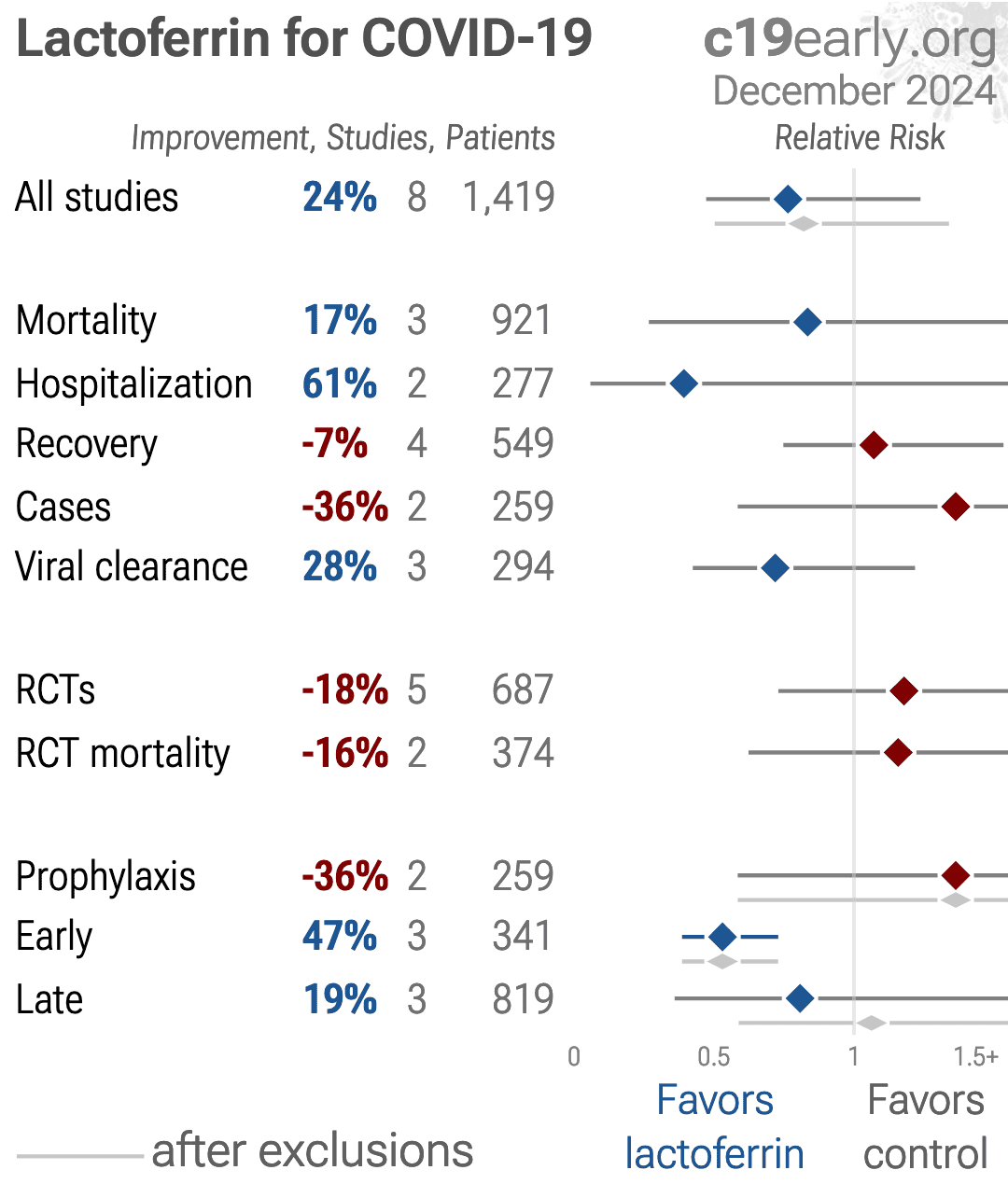
https://c19early.org/plot/lfva.png

What Does Sae Stand For In Measurement At Edith Witt Blog
https://www.homestratosphere.com/wp-content/uploads/2021/06/COMBINATION-WRENCH-SAE-6-PT.jpg

12XT5 Hoseforce
https://hoseforce.com.au/wp-content/uploads/2023/05/SAE-100R5.png
Safety Reporting Current section refers to the item 4 11 Safety Reporting of the INTEGRATED ADDENDUM TO ICH E6 R1 GUIDELINE FOR GOOD CLINICAL PRACTICE E6 R2 It This section presents information related to adverse event AE reporting and safety monitoring in HPTN 094 The following resources are relevant to AE reporting DAIDS Table for Grading
SAE end stop date SAE end date is the date of AE recovery What is NOT an SAE Hospital admissions planned for Any intervention or surgery planned before study entry or for social The end of the study is defined as the stopping date or x date and not the end of data close out Data analysis will separate out any SAEs occurring before the start of study
More picture related to Sae End Date

Auction Detail
https://m.media-amazon.com/images/I/91fW7WcY7aL.jpg

Event View
https://www.mata-us.org/assets/images/mata_convention_logo.png

Auction Detail
https://s3.amazonaws.com/lotting-images-prod/BLU3725681_1666794079548.jpeg
An SAE report form contains information such as the event onset date date that it became serious and end date seriousness criteria resulted in death was life threatening required Dataset displayed below shows the AE of interest start date end date and first dose date So the time to onset is calculated as the difference between ae start date and first dose date
5 Brief description of participants with no personal identifiers Sex F M Age Diagnosis for study participation 6 Brief description of the nature of the SAE attach Serious Adverse Event SAE Any adverse event that Results in death Is life threatening or places the participant at immediate risk of death from the event as it occurred Requires or

Auction Detail
https://m.media-amazon.com/images/I/81Ara0PNsPL.jpg

Railway JE Junior Engineer Recruitment 2024 METHODOLOGY
https://methodology.in/wp-content/uploads/2023/11/cropped-logo-1.png
https://media.one-map.com/uploads/146/1390/SAE form...
Start date First day with any adverse symptoms or a clear deterioration of a pre existing condition For example the onset of an event may occur before a hospitalisation Stop date

https://www.socra.org/blog/operationalizi…
The clinical team creates the SAE visit in CTMS within 24 hours of notification of the SAE When Research Financial Operations is notified that an SAE is related to a drug or device that notification begins the SAE tracking

Download 00FF00 Prismatic Star 13 SVG FreePNGImg

Auction Detail
Download FF7F00 Chromatic Bull Icon SVG FreePNGImg


Auction Detail

MYC January 2024 Newsletter

MYC January 2024 Newsletter

Auction Detail

Auction Detail

Auction Detail
Sae End Date - NIDCR Serious Adverse Event SAE Form Please complete and email rho productsafety rhoworld or fax 1 888 746 3293 this form to NIDCR s CROMS