What Is Another Name For Sweet Potato Vine Keep reading to learn how FDA EU and WHO GMP guidelines compare where global harmonization is heading and how to keep your compliance strategy sharp across
Under section 520 f of the Act FDA issued a final rule in the Federal Register of July 21 1978 43 FR 31 508 prescribing CGMP requirements for medical devices This Abstract This chapter describes the good manufacturing practice GMP for medical devices It first reviews the history of GMP and essential prerequisites as well as the main
What Is Another Name For Sweet Potato Vine
![]()
What Is Another Name For Sweet Potato Vine
https://shop.madhusudanmasala.com/wp-content/uploads/2023/03/favicon-eng.png

Sweet Potato Flower Top Facts Tips
https://www.garden.eco/wp-content/uploads/2018/07/sweet-potato-leaves-1020x680.jpg

Dextrose Now Foods Canada
https://nowfoods.ca/wp-content/uploads/2022/03/NOW06925_01-1000x1000.png
By understanding the regulations establishing a robust QMS and fostering a culture of quality you can navigate the path to GMP compliance and achieve success in the On 76 pages spread over five chapters the WHO has published a finalised regulatory framework for medical devices including In vitro Diagnostics IVDs with the title
Compliance with GMP ensures that medical devices are safe effective and meet quality standards Failure to comply can result in regulatory actions including recalls and legal Following implementation of these WHO good manufacturing practices GMP guidelines 1 within the context of the WHO Prequalifi cation of Medicines Programme clarifying editorial
More picture related to What Is Another Name For Sweet Potato Vine

Tensile Cone Structure Manufacturer Smarttensileroofing
https://smarttensileroofing.in/assets/images/logo.png

No Place To Play Exhibit Explores How Gender Shapes Teens Access To
https://news.clemson.edu/wp-content/uploads/2023/03/popart.postcard-1024x680.png

Mary Jane Sullivan Gardening
https://i.pinimg.com/originals/a1/5e/ef/a15eef3e131358ff97ab46f6638e6b90.jpg
Good Manufacturing Practices GMP are indispensable in the medical device industry ensuring that products are consistently produced and controlled according to stringent quality standards Soon afterward FDA began to actively pursue the harmonization of GMP requirements on a global basis Over the following two years FDA took steps to ensure that
[desc-10] [desc-11]

Sweet Potato Vine Central Texas Gardener
https://www.centraltexasgardener.org/wp-content/uploads/2014/08/pow-sweet-potato-vine-1024x768.jpg
Ask A Doctor What Does The Name Anasarca Mean
https://img-s-msn-com.akamaized.net/tenant/amp/entityid/AA1jnqh5.img?w=4206&h=2804&m=4&q=71
https://www.cfpie.com › global-gmp-standards...
Keep reading to learn how FDA EU and WHO GMP guidelines compare where global harmonization is heading and how to keep your compliance strategy sharp across

https://www.fda.gov › medical-devices › postmarket...
Under section 520 f of the Act FDA issued a final rule in the Federal Register of July 21 1978 43 FR 31 508 prescribing CGMP requirements for medical devices This
:max_bytes(150000):strip_icc()/sweet-potato-vines-4120149-hero-0cefde8567fd4a1083c094fd4b7aff0c.jpg)
Potato Plant Leaves

Sweet Potato Vine Central Texas Gardener
:max_bytes(150000):strip_icc()/2547601_junec_0192-2000-e2997131dc3e44bf9b545dbbaf2fb1df-5636c1aaef7f4931931d05fa79d49b84.jpg)
Potted Potato Plant
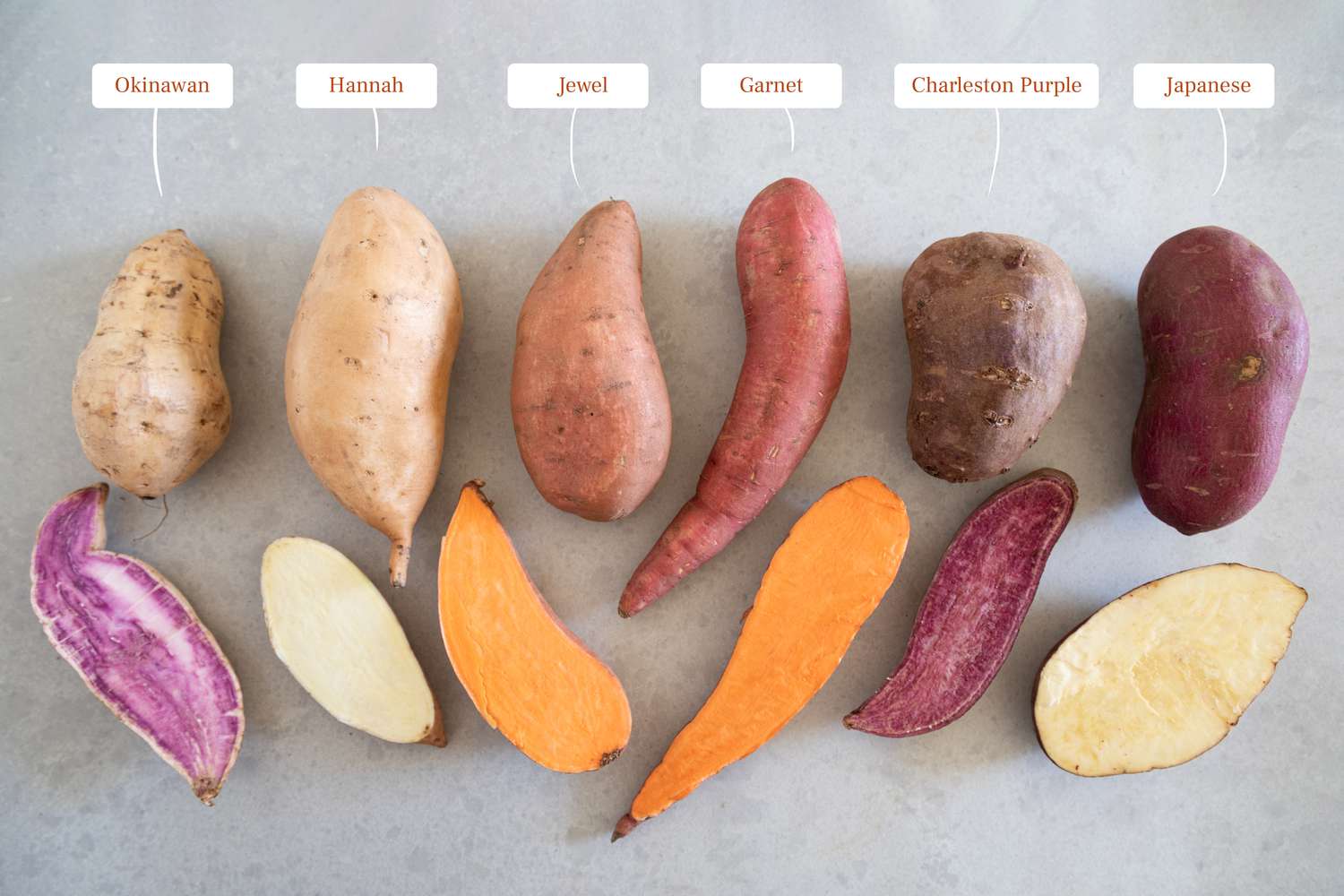
8 Types Of Sweet Potatoes Uses Of Sweet Potato

3 pack Hair Clips White Kids H M US

Creative Sweet Potato Vine Growing Tips And Ideas Empress Of Dirt

Creative Sweet Potato Vine Growing Tips And Ideas Empress Of Dirt

What Is The Opposite Of Sweet Antonyms Sweet Promova

Sweet Potato Houseplant

Creamy Garlicky Sweet Potato Fries Dipping Sauce Real Simple Good
What Is Another Name For Sweet Potato Vine - Following implementation of these WHO good manufacturing practices GMP guidelines 1 within the context of the WHO Prequalifi cation of Medicines Programme clarifying editorial
