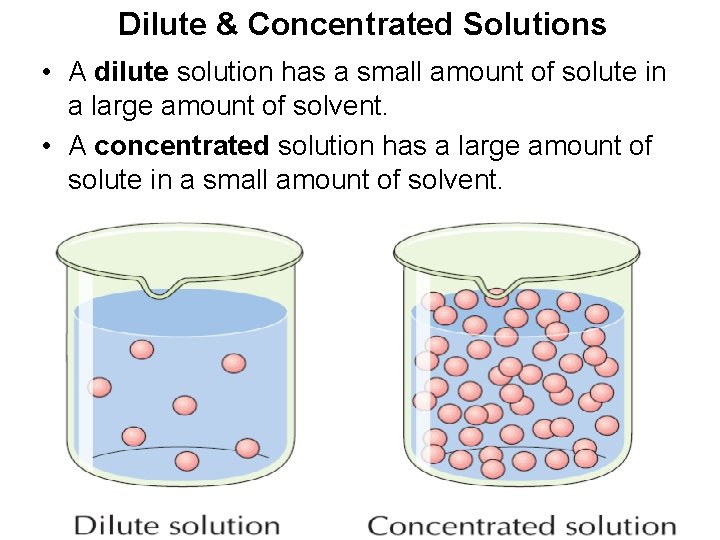
What Is A Dilute Solution Solution If the solution has more amount of solute which is comparable to solvent the solution is said to be concentrated solution while if the amount of solute is very less as compared to the
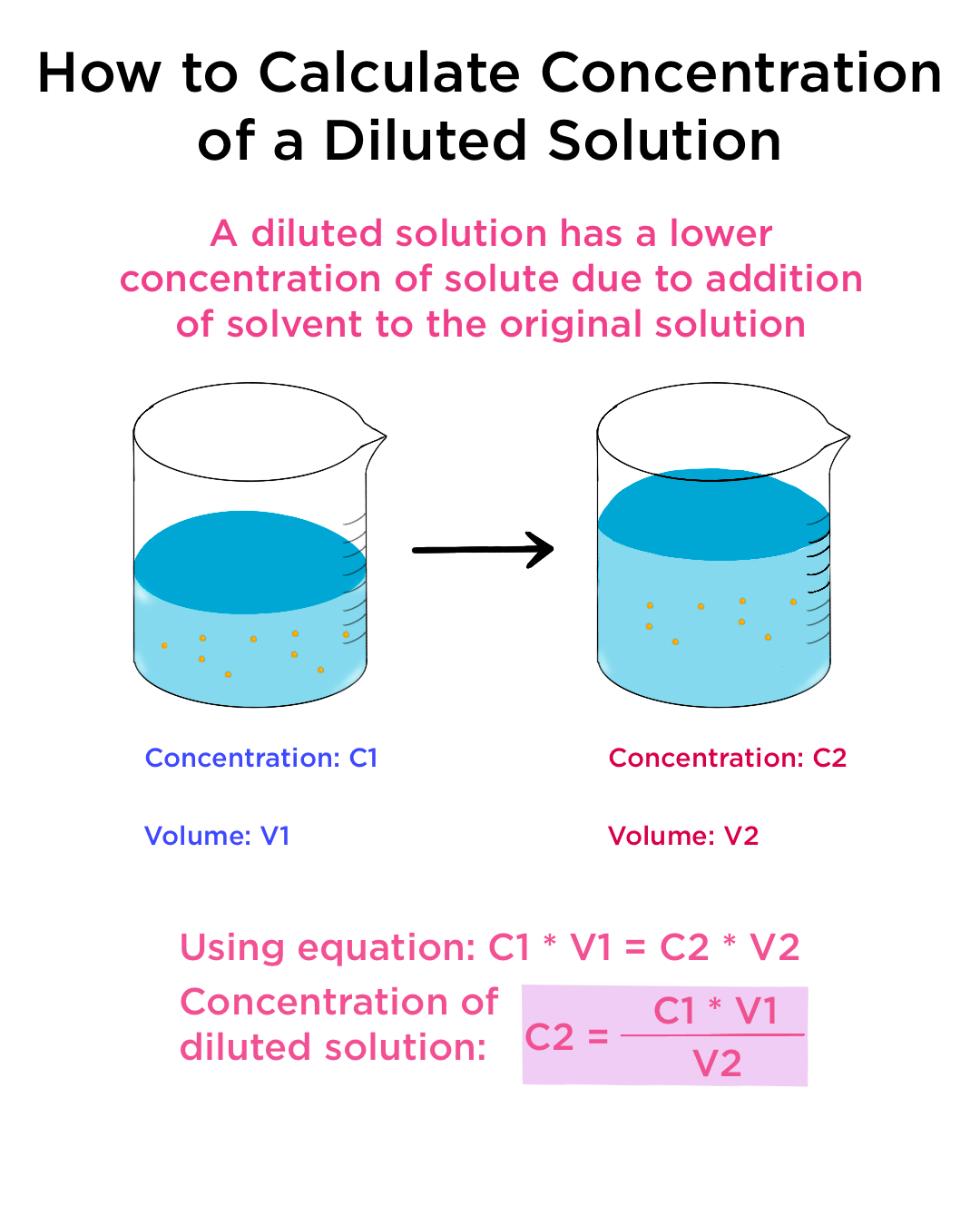
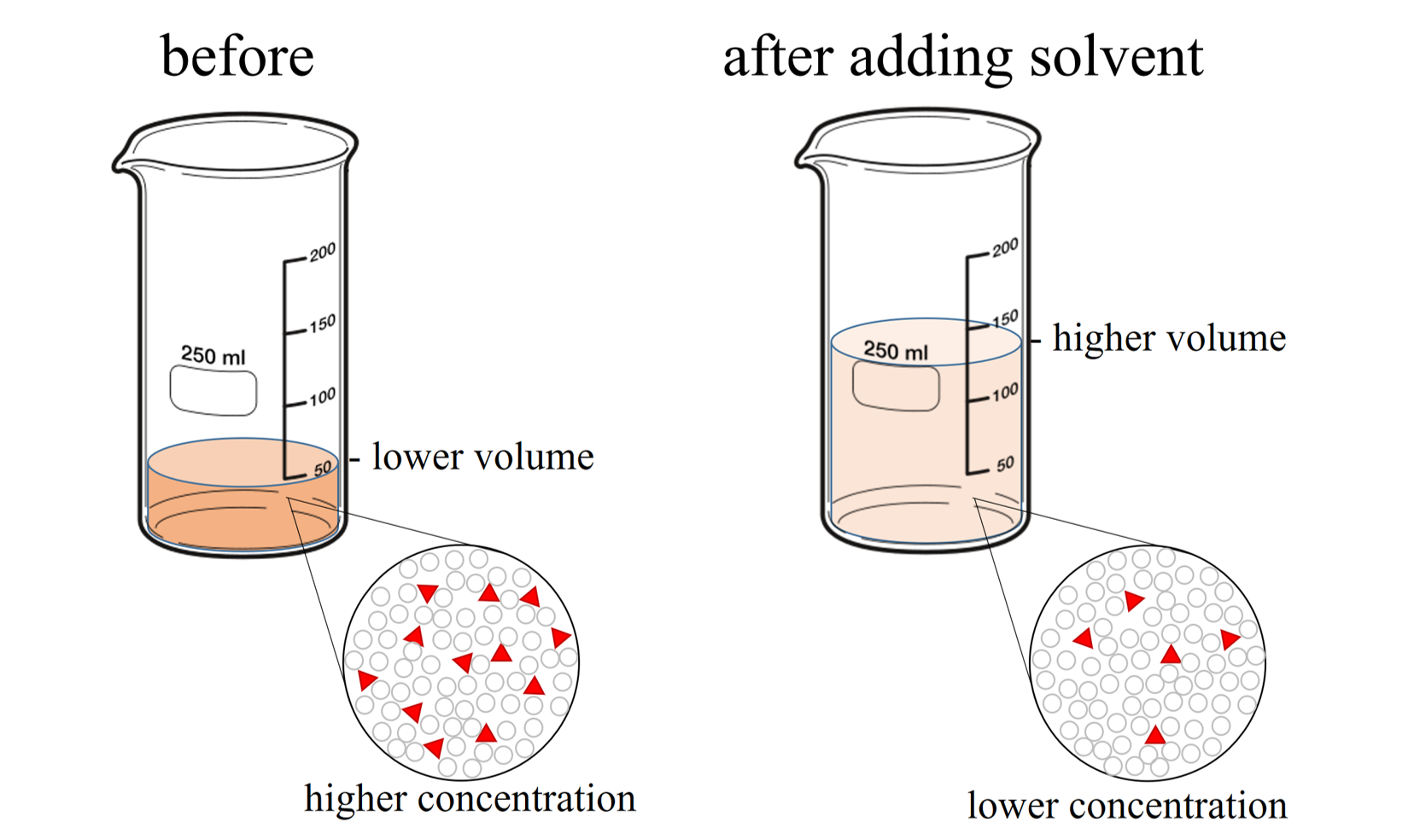
Dilution is the process of reducing the concentration of a solute in solution usually simply by mixing with more solvent Example 1 You can add water to concentrated orange juice to dilute Dilute solution Concentrated solution A solution where the concentration of solute is low is a dilute solution A solution where the concentration of solute is high is a concentrated solution
What Is A Dilute Solution
What Is A Dilute Solution
https://d20khd7ddkh5ls.cloudfront.net/dilution.png

What Is Dilute Solution Chemistry YouTube
https://i.ytimg.com/vi/eVhJnFpRqG0/maxresdefault.jpg

Dilution When Solvent Is Added To Dilute A Solution The Number Of
https://i.pinimg.com/originals/b5/f9/04/b5f904fc793680d474f6351e36ac239c.jpg
Homogenous and Heterogeneous Solutions Homogeneous solutions are solutions with uniform composition and properties throughout the solution For example a cup of coffee perfume The dilute solution still has 10 grams of salt To prepare a fixed amount of dilute solution we have a formula C1V1 C2V2 Where V1 denotes the Volume of stock solution needed to make
A dilute solution means the quantity of solute is relatively very small and a concentrated solution implies that the solution has a large amount of solute But these are relative terms and do not Q1 From the below options choose the correct example for gaseous solutions a Oxygen dissolved in water b Camphor in nitrogen gas c Carbon dioxide dissolved in water d
More picture related to What Is A Dilute Solution

How To Dilute Solutions 8 Steps with Pictures WikiHow
http://www.wikihow.com/images/a/ac/Dilute-Solutions-Step-4.jpg
How Does A Dilute Solution Work At Annie Ahmed Blog
https://slidetodoc.com/presentation_image/b943283d44b67f61a486e88fe34057f6/image-8.jpg

Science Chemistry Solubility Dilution Fundamental Photographs The
https://ssl.c.photoshelter.com/img-get/I0000acHwM5Wmehw/s/860/860/Fphoto-64780908D-6RM.jpg
Notably when we talk about the concentrations of the solutions most of the dilute solutions also have or show characteristics of an ideal solution FAQs 1 What do you mean by an ideal Wash rinse and fill the burette with M 10 Na 2 CO 3 solution Note the initial reading Take 10cm 3 of HCl solution with the help of a pipette and transfer it into a clean washed titration flask
[desc-10] [desc-11]

What Is The Difference Between A Dilute Solution And A Concentrated
https://ph-static.z-dn.net/files/d79/d697764f4e653952d36bc45bb69affbf.jpg

Dilute Flashcards Easy Science Science Student
https://i.pinimg.com/originals/18/65/f7/1865f7b0f9ca87bde2518ab10873d191.png

https://byjus.com/question-answer/what-is-dilute-solution
Solution If the solution has more amount of solute which is comparable to solvent the solution is said to be concentrated solution while if the amount of solute is very less as compared to the

https://byjus.com/question-answer/definition-of-dilute-solution
Dilution is the process of reducing the concentration of a solute in solution usually simply by mixing with more solvent Example 1 You can add water to concentrated orange juice to dilute
14 7 Solution Dilution Chemistry LibreTexts

What Is The Difference Between A Dilute Solution And A Concentrated
SOLVED Which Gas Is Produced When Zinc Granules React With Dilute

How Can A Solution Be Dilute Or Concentrated Brainly in

How To Prepare Dilute Solution Of Sulphuric Acid H2SO4 In Laboratory
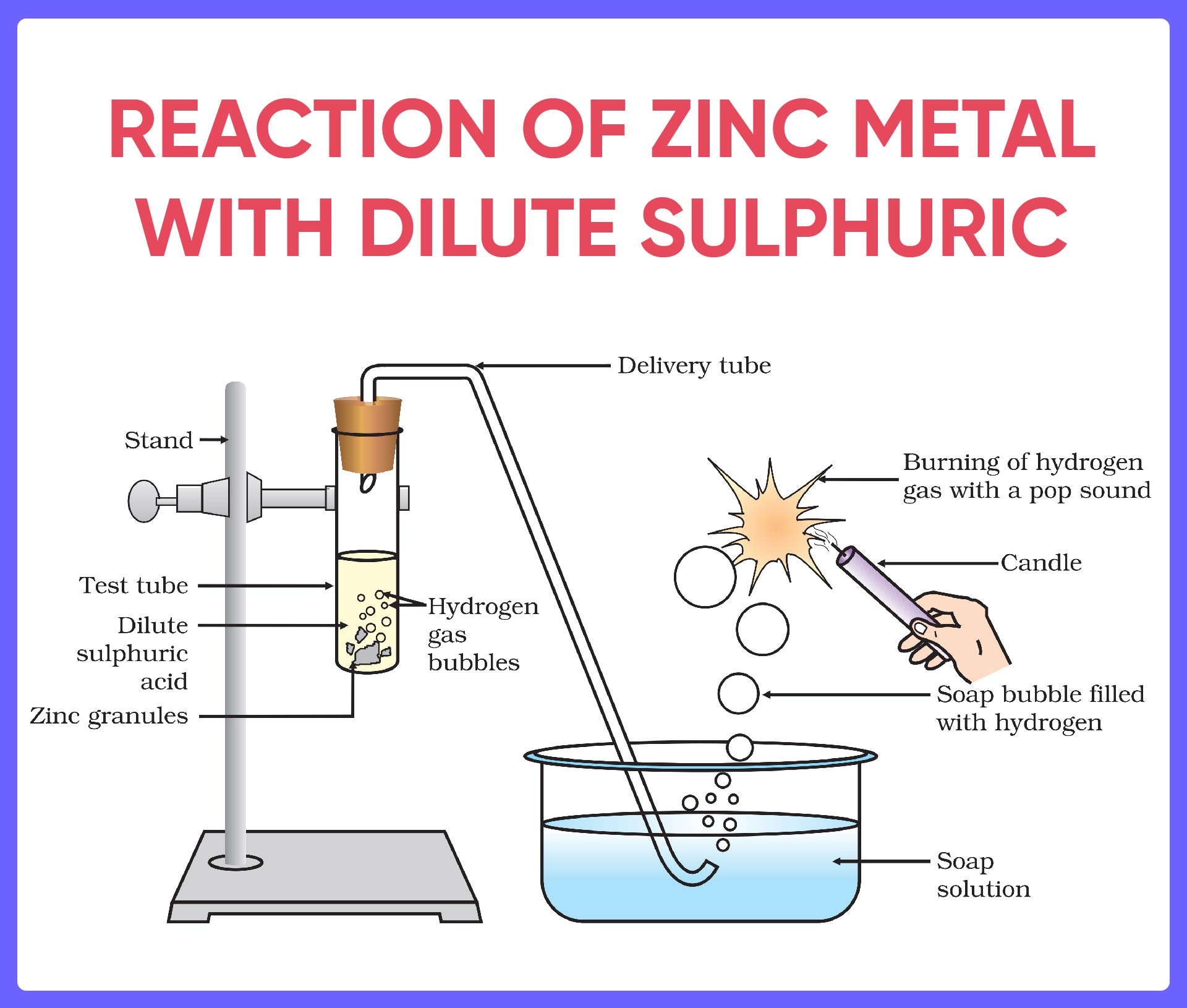
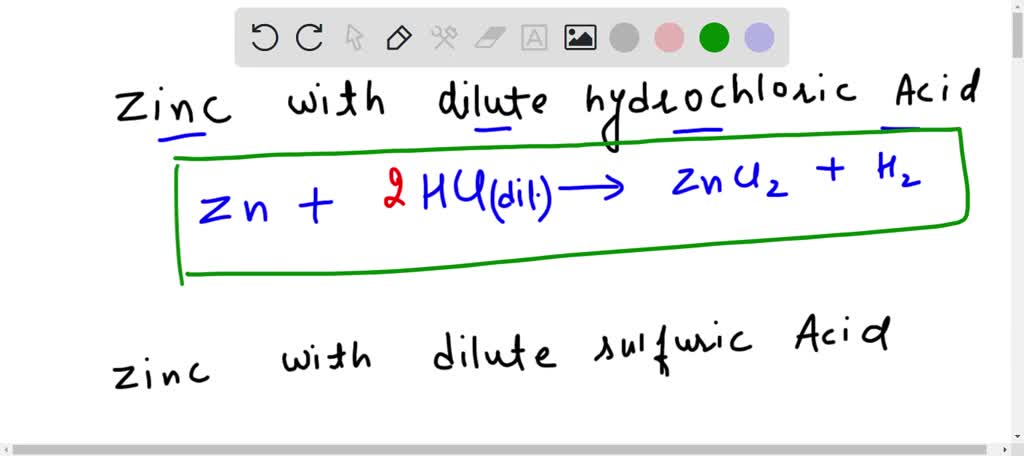
Reactions Of Acids And Bases Full List with Examples Teachoo

Reactions Of Acids And Bases Full List with Examples Teachoo

How To Prepare Dilute Solution From Concentrated Acid Laboratory

Serial Dilution Vs Parallel Dilution
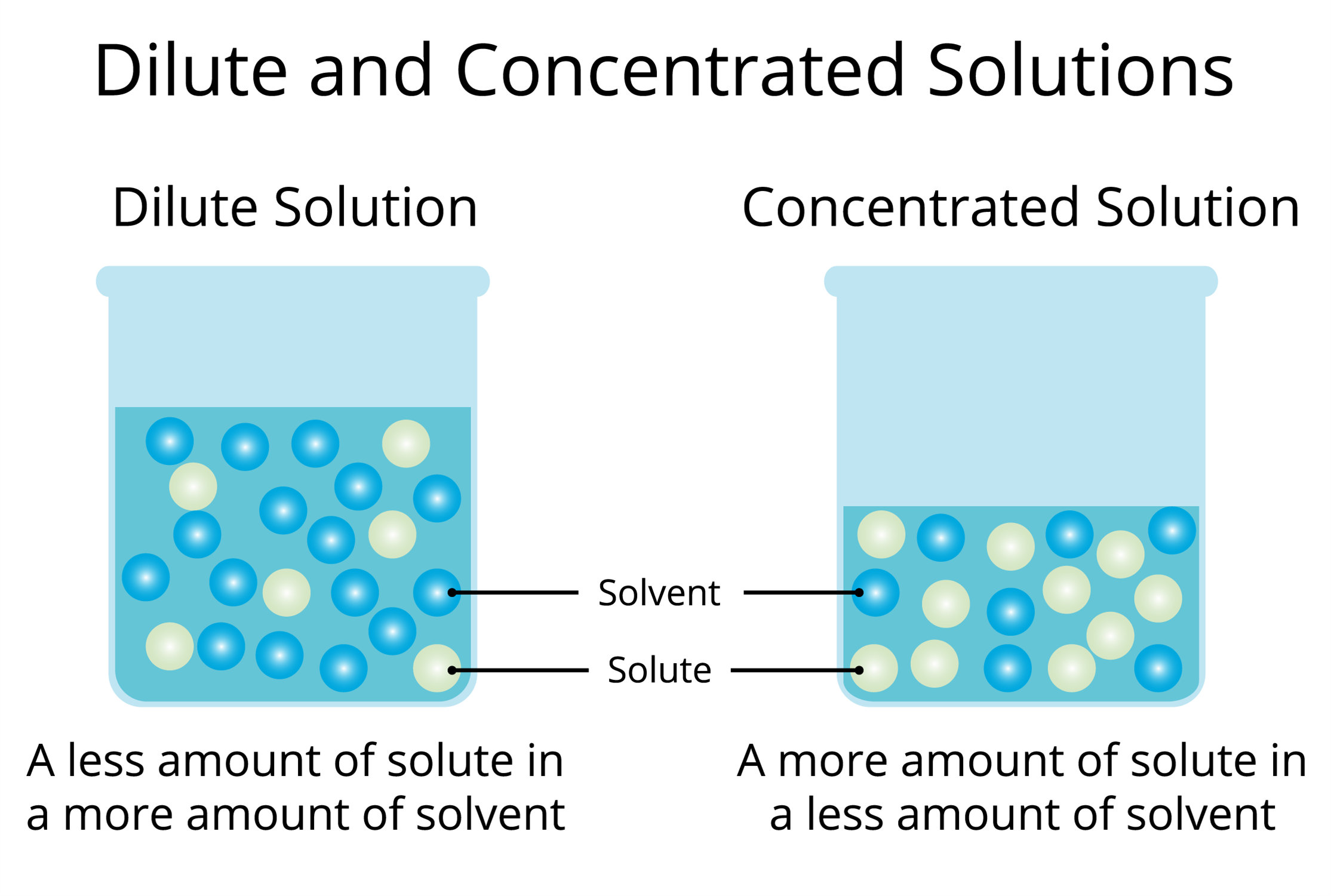
Types Of Solutions Concentrated And Dilute Solutions Lesson Science
What Is A Dilute Solution - A dilute solution means the quantity of solute is relatively very small and a concentrated solution implies that the solution has a large amount of solute But these are relative terms and do not